Unlearn
Open siteHealth & Wellness
Introduction
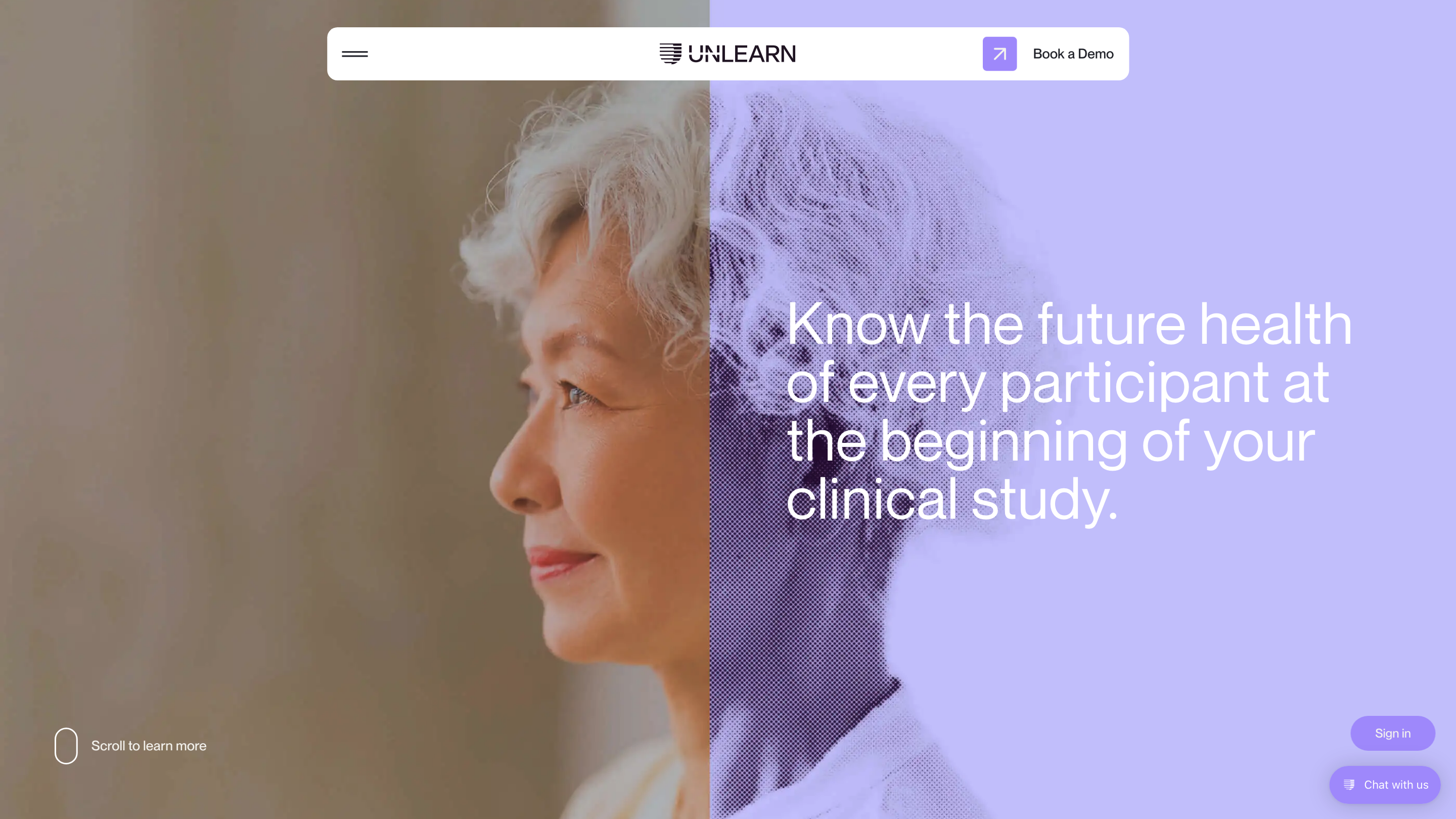
Advancing AI to power the future of medicine
Unlearn Product Information
Unlearn Platform – Digital Twin Generators for Clinical Trials is an AI-driven platform that creates digital twins of patients using clinical trial data to predict outcomes at every future time point. The goal is to minimize trial failures, reduce costs, and accelerate regulatory approval by providing precise, real-time insights and scenario testing across subgroups. The platform emphasizes ease of use, interoperability with neuroscience, immunology, metabolic disease research, and other therapeutic areas, and highlights cost savings and arm-size optimization as key outcomes.
How it works
- Build digital twins of trial participants from existing clinical data.
- Predict outcomes for any time point and any subgroup, enabling real-time, data-driven decision making throughout the study.
- Use digital twins during trial planning to optimize endpoints, endpoints, and composite scores with enhanced sensitivity at both population and subpopulation levels.
- Apply the platform to accelerate development, reduce control-arm size, and lower overall trial costs.
Solutions and Benefits
- Accelerate Timelines with Smaller, More Efficient Studies: Design and run smaller trials that maintain power or more powerful studies without additional participants. This approach is aligned with EMA guidance and FDA expectations.
- Enhance Decision-Making with Digital Twins: Explore predicted outcomes for any subgroup of participants for all measured clinical variables at any time point.
- Identify Sensitive Clinical Outcomes: Generate participants’ digital twins resembling the target population during planning to optimize study endpoints and composite scores.
- Control Arm Size Reduction: Estimated cost savings of significant magnitude (illustrative figure: 33% arm-size reduction; $50M in estimated cost savings).
- Accelerating Clinical Development in Alzheimer's Disease and Beyond: Applicable across neuroscience, immunology, metabolism, and more.
Highlights and Resources
- Case studies and demos available to illustrate impact
- Regular updates on AI innovation in clinical research
- Thought leadership and industry commentary related to digital twins and AI in healthcare
How to Get Started
- Book a demo to discuss study goals and how the Unlearn Platform can support your trial
- Explore platform capabilities, data requirements, and integration with existing clinical trial systems
- Review potential ROI, timelines, and implementation considerations
Platform Areas
- Digital Twin Generators
- Research and Artificial Intelligence modules
- Clinical Research workflow integration
- Real-time analytics and subgroup analysis
Safety and Compliance Considerations
- Uses de-identified or appropriately consented clinical data to generate predictive twin models
- Adheres to relevant regulatory guidelines for AI in clinical trials and data privacy
Core Features
- AI-driven digital twin generation from clinical trial data
- Predict outcomes for all measured variables at any time point
- Real-time insights for decision-making throughout the study
- Subgroup and population-level analyses with enhanced sensitivity
- Arm-size reduction and cost-saving potential
- Support for multiple therapeutic areas (neuroscience, immunology, metabolic disease, etc.)
- Demo-ready case studies and enterprise-ready deployment
- Regulatory-aligned design and EMA/FDA-aligned guidance
- Easy-to-use platform interface with integration capabilities